Vaccinia virus Recombinants
Description
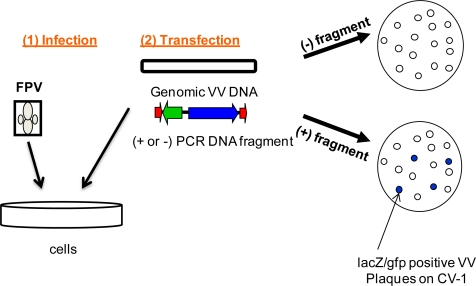
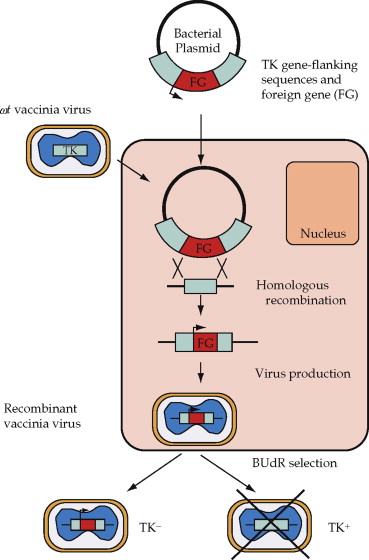
Recombinant vaccinia virus is produced by homologous recombination between a plasmid and viral DNA within infected cells. The gene of interest is inserted at a nonessential locus in vaccinia DNA or between two essential genes to minimize unstable recombinants. To aid in the recovery of the recombinant virus, plasmids have been generated that allow the selection of the recombinant virus.
Since vaccinia encodes its own DNA-dependent RNA polymerase, which does not recognize foreign promoters, the cDNA must be cloned downstream of a well-defined virus promoter or close to an endogenous vaccinia promoter.
Vaccinia viruses are widely used for the study of poxvirus molecular biology, functional characterization, and protein overexpression, and as candidates for live vaccines or vaccine research.
Check the security considerations required by your institution. Many institutions require smallpox vaccination prior to use for wild-type vaccinia, but not for MVA (Modified Vaccinia Ankara).
1. Amplification, purification and titration of viral particles
Provided material: 8 ml of the purified virus at a concentration ranging between 1 x107 pfu/ml and 1 x109 pfu/ml.
Required sample: Viral particles
Timeline: 3-4 weeks from the moment the virus is received.
Services include:
- large-scale amplification
- Sucrose cushion purification
- An infectious titer (pfu/mL) is provided with each new lot.
Vehicle: Vaccinia vectors are resuspended in 1 mM Tris HCl, pH 9.0.
Wild type cost: $2860 build plus shipping and handling.
Cost per MVA: $3,080 for construction plus shipping and handling.
2. Construction, selection, purification, amplification and titer
Provided material: 8 ml of the purified virus at a concentration ranging between 1 x107 pfu/ml and 1 x109 pfu/ml.
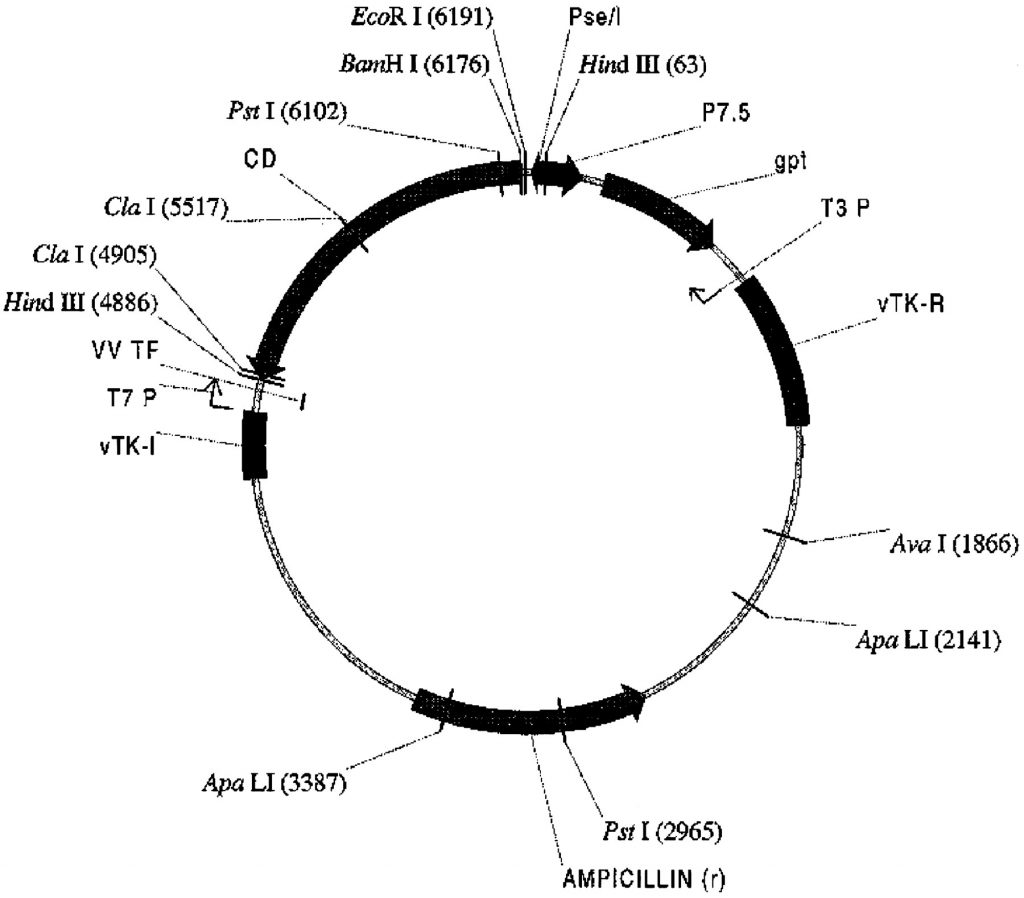
Required sample: 20 µg of vaccinia shuttle plasmid expressing the gene of interest at a concentration greater than 0.25 µg/µl.
Timeline: 6-8 weeks from the time the plasmid is received.
Services include:
- transfection
- Cell sorting using a fluorescent marker for faster isolation of recombinants (MVA only)
- Three or more rounds of selection depending on the choice of shuttle plasmid
- large-scale amplification
- Sucrose cushion purification
- An infectious titer (pfu/mL) is provided with each new lot.
Vehicle: Vaccinia vectors are resuspended in 1 mM Tris HCl, pH 9.0.
Wild type cost: $3500 build plus shipping and handling.
MVA cost: $3,700 for construction plus shipping and handling.
